20 / 10 / 16
医学翻译学习-肝细胞癌
关于“肝细胞癌”一文医学翻译摘要学习情况,记录于此。
肝细胞癌占原发性肝癌的大多数。
Hepatocellular carcinoma accounts for the majority of primary liver cancers.
笔记:
- 原发性肝癌 primary liver cancer
在全球范围内,肝癌是癌症相关死亡的第四大原因,在新发病例数量方面排名第六1 。
Worldwide, liver cancers are the fourth most common cause of cancer-related death and rank sixth in terms of incident cases .1
笔记:
- 用的是并列句,平衡重点
根据年度预测,世界卫生组织估计,2030年将有超过100万患者死于肝癌2 。
On the basis of annual projections, the World Health Organization estimates that more than 1 million patients will die from liver cancer in 2030.2
笔记:
- annual projections 年度预测
- die from
在美国,2000—2016年,肝癌死亡率增加了43%(从每10万人中有7.2人死亡增加至10.3人死亡)3 。
In the United States, the rate of death from liver cancer increased by 43% (from 7.2 to 10.3 deaths per 100,000) between 2000 and 2016.3
笔记:
- the rate of death from xxx xxx的死亡率
肝癌的5年生存率为18%,是胰腺癌之后排名第二的致死性肿瘤4 。
With a 5-year survival of 18%, liver cancer is the second most lethal tumor, after pancreatic cancer.4
笔记:
- lethal tumor 致死性肿瘤
大多数肝细胞癌发生于有基础肝病的患者,主要是由乙型或丙型肝炎病毒(HBV or HCV)感染或者酒精滥用导致。
The majority of hepatocellular carcinomas occur in patients with underlying liver disease, mostly as a result of hepatitis B or C virus (HBV or HCV) infection or alcohol abuse.
笔记:
-
patients with underlying liver disease 有基础肝病的患者
-
结构学习:mostly as a result of
HBV疫苗的普遍接种及抗HCV的直接抗病毒药的广泛应用可能改变肝细胞癌的病因学格局。
Universal HBV vaccination and wide implementation of direct-acting antiviral agents against HCV are likely to change the etiologic landscape of hepatocellular carcinoma.
然而,非酒精性脂肪性肝病的增多与代谢综合征和肥胖一起增加了肝癌的风险,非酒精性脂肪性肝病将很快成为西方国家肝癌的主要原因5 。
However, the increase in nonalcoholic fatty liver disease (NAFLD), which together with metabolic syndrome and obesity amplifies the risk of liver cancer, will soon become a leading cause of liver cancer in Western countries.5
笔记:
- nonalcoholic fatty liver disease (NAFLD) 酒精性脂肪性肝病
- metabolic syndrome 代谢综合征
- the increase in
- together with/in addition to; as well as
- 个人觉得将“非酒精性脂肪性肝病”重复两遍,可以再优化一下。此外,which接的非限制性定从,是否可以不要处理的让人觉得这句话很重要呢....
人种和族群差异在生存概率方面起着重要作用,黑种人和西班牙语裔接受根治的可能性比白种人低6 。
Racial or ethnic group differences play an important role in the probability of survival, with blacks and Hispanics less likely than whites to undergo curative therapies.6
晚期肝癌患者的全身性治疗正在迅速发生改变,过去2年中有4种新药在3期试验中显示出临床疗效7 。
Systemic therapies for patients with an advanced stage of liver cancer are rapidly changing, with four new agents showing clinical efficacy in phase 3 trials in the past 2 years.7
笔记:
- with+v-ing的这种结构实在是很有意思!
- an advanced stage of liver cancer
本文综述了肝细胞癌的主要基因改变、重要流行病学特征和循证治疗方法。
This review summarizes the main genetic alterations in hepatocellular carcinoma, key epidemiologic features, and evidence-based approaches to management.
笔记:
- approaches to 介词是很关键的
- 牛津逗号深得我心
分子发病机制
Molecular Pathogenesis
慢性肝病患者持续存在肝脏炎症、纤维化和异常肝细胞再生。
Patients with chronic liver disease have sustained hepatic inflammation, fibrosis, and aberrant hepatocyte regeneration.
笔记:
- 动词啊动词,永远会发现英语的美
这些异常可导致肝硬化,并促进一系列遗传和表观遗传事件的发生,最终形成发育不良结节,而发育不良结节是真正的瘤前病变。
These abnormalities can cause cirrhosis and favor a series of genetic and epigenetic events that culminate in the formation of dysplastic nodules, which are bona fide preneoplastic lesions.
笔记:
- favor这个动词,来了解下:
to provide suitable conditions for a particular person, group, etc. 有助于;有利于
- 这篇文章译者对于英文代词很喜欢再次重复全称呢...
其他分子改变为发育不良细胞提供了增殖、侵袭和生存优势,并完成了向完全肝细胞癌的转变(图1)8 。
Additional molecular alterations provide dysplastic cells with proliferative, invasive, and survival advantages and complete the transition to full-blown hepatocellular carcinoma (Figure 2 ).8
肝细胞癌也可发生于患慢性肝病但未形成肝硬化或明显炎症的患者(如HBV感染者)。
Hepatocellular carcinoma can also arise in patients who have chronic liver disease but do not have established cirrhosis or marked inflammation (e.g., patients with HBV infection).
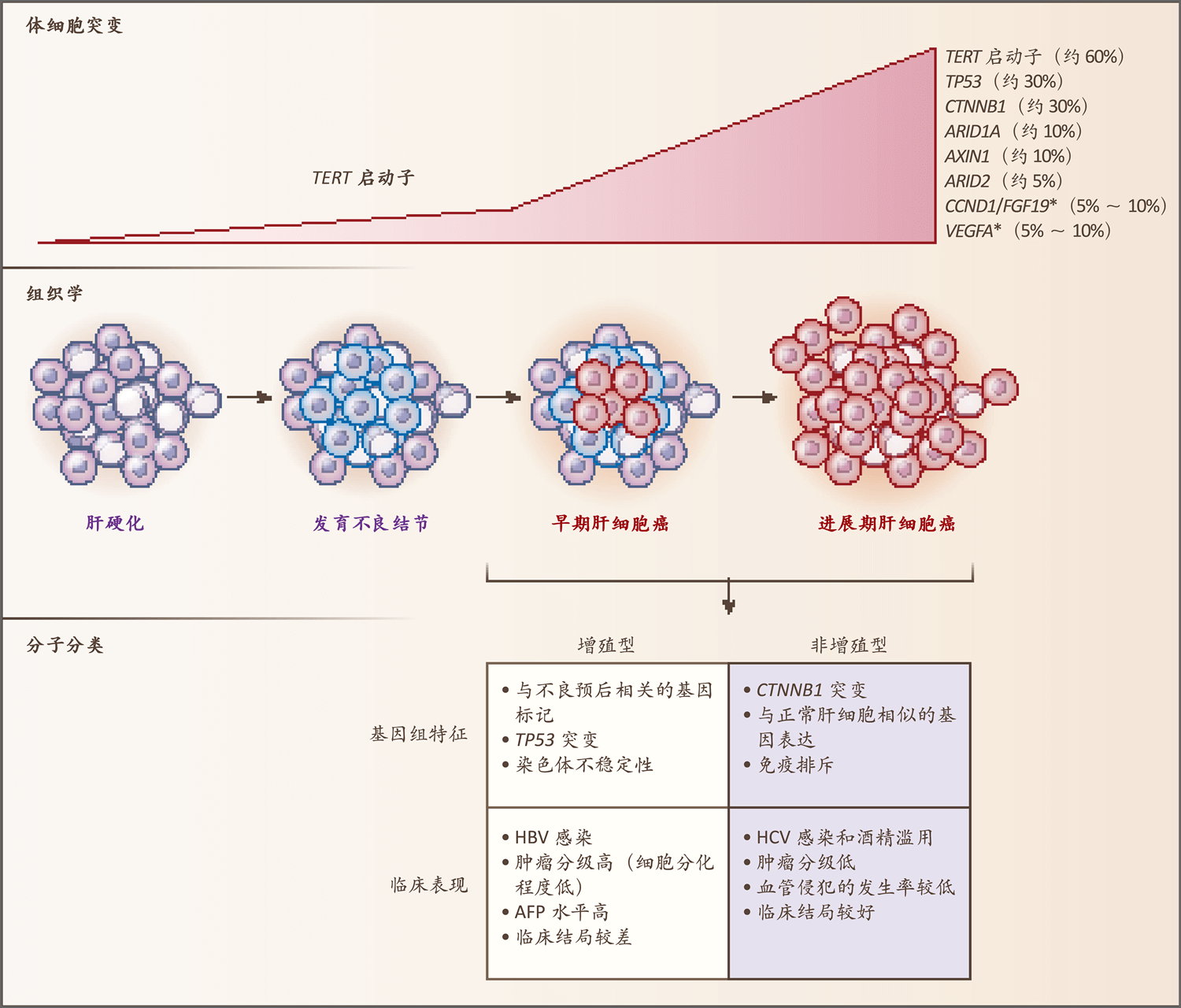
图1. 肝细胞癌的主要基因改变和分子分类
Figure 1. Main Genetic Alterations in Hepatocellular Carcinoma and Molecular Classification.
笔记:
- 基因改变 genetic alteration
图中总结了人肝癌发生过程中的关键分子和组织学变化,以及肝细胞癌两个主要分子亚型的主要基因组特征和临床表现。
The key molecular and histologic alterations occurring during human hepatocarcinogenesis are summarized, as are the main genomic and clinical features of the two main molecular subclasses of hepatocellular carcinoma.
笔记:
- 句子结构很有意思
星号(*)表示高水平DNA扩增。
An asterisk (*) denotes high-level DNA amplification.
在非增殖型中,免疫排斥在CTNNB1突变的肿瘤内富集。
In the nonproliferation class, immune exclusion is enriched in tumors with CTNNB1 mutations.
笔记:
- nonproliferation class 非增殖型
- enrich 富集
AFP表示甲胎蛋白、HBV表示乙型肝炎病毒,HCV表示丙型肝炎病毒。
AFP denotes alpha-fetoprotein, HBV hepatitis B virus, and HCV hepatitis C virus.
笔记:
- 甲胎蛋白 alpha-fetoprotein
- 常见的省略动词结构
基因改变
GENETIC ALTERATIONS
肝细胞癌细胞累积体细胞DNA改变,包括突变和染色体畸变。
Hepatocellular carcinoma cells accumulate somatic DNA alterations, including mutations and chromosomal aberrations.
笔记:
- 累积 accumulate
TERT启动子突变是最常见的基因改变,在所有病例中约占60%9 。
Mutations in the TERT promoter are the most frequent genetic alterations, accounting for approximately 60% of cases.9
笔记:
- TERT promoter TERT启动子
它们可在发育不良结节内检出,TERT启动子是HBV基因组的重复插入位点。
They can be detected in dysplastic nodules, and the TERT promoter is a recurrent insertion site for the genome of HBV.
笔记:
- recurrent insertion site 重复插入位点
也有报道称TERT启动子内插入了致癌的2型腺相关病毒,但其发生的比率非常低(约5%)9 。
Oncogenic viral insertions of adeno-associated virus type 2 in the TERT promoter have also been reported, but their prevalence is very low (approximately 5%).9
其他突变基因影响细胞周期(例如TP53,在所有病例中约占30%)、WNT信号传导(CTNNB1和AXIN1,分别约占30%和10%)或染色质重塑(ARID1A和ARID2,分别约占10%和5%)(图1)。
Other mutated genes affect the cell cycle (e.g., TP53, accounting for approximately 30% of cases), WNT signaling (CTNNB1 and AXIN1, accounting for approximately 30% and 10% of cases, respectively), or chromatin remodeling (ARID1Aand ARID2, accounting for approximately 10% and 5% of cases, respectively) (Figure 1 ).
肝细胞癌内可作为分子疗法靶点的体细胞突变很少,属于最少的几种实体癌之一10 ,临床实践中尚无用于预测疗效的突变。
Hepatocellular carcinoma is among the solid cancers with the fewest somatic mutations that can be targeted with molecular therapies,10 and no mutation is used in clinical practice to predict a therapeutic response.
笔记:
- 没有强行there be,很开心
位于染色体6p21和11q13(分别为VEGFA和CCND1/FGF19的基因座)的高水平DNA扩增也可作为分子疗法靶点,但其发生的比率低。
High-level DNA amplifications located in chromosome 6p21 and 11q13, the respective loci for VEGFAand CCND1/FGF19, could also be targeted with molecular therapies, but their prevalence is low.
笔记:
- 英文插入语,中文处理为了括号。常见方式,很不错。
分子分类和生物标志物
MOLECULAR CLASSIFICATION AND BIOMARKERS
同一临床分期的肝细胞癌患者可以有不同的分子亚型。
Patients with hepatocellular carcinoma at the same clinical stage can have different molecular subtypes.
这些亚型与临床表现相关,但常规临床实践中并未应用。
These subtypes correlate with clinical features, but they are not used in routine clinical practice.
笔记:
- 常规临床实践 routine clinical practice
它们是在早期疾病患者切除标本的基础上鉴定出,但未全面检测其可否作为全身性治疗效果的预测因素,因此限制了临床应用。
They were identified on the basis of specimens resected from patients at early disease stages but have not been thoroughly tested as predictors of a response to systemic therapies, which has limited their clinical usefulness.
笔记:
- 英文美显示的淋漓尽致...各种介词逻辑结构,很过瘾....不过个人更喜欢把最终的结论放在前面....说好的英文先表态后事实嘛....(逃
分子亚型可以分为两大类:增殖型和非增殖型(图1)11 。
The molecular subtypes can be grouped in two main classes: the proliferation class and the nonproliferation class (Figure 2 ).11
增殖型更常见于HBV感染者,其典型特征是导致侵袭性临床行为的分子和组织学特征,包括血清甲胎蛋白水平高、细胞分化程度低、染色体不稳定、TP53突变和致癌通路激活(例如RAS-丝裂原活化蛋白激酶[MASK]、AKT -哺乳动物雷帕霉素靶蛋白[mTOR]和MET[肝细胞生长因子受体])。
The proliferation class, more commonly seen in patients with HBV infection, is characterized by molecular and histologic features that result in aggressive clinical behavior, including high serum levels of alpha-fetoprotein, poor cell differentiation, chromosomal instability, TP53 mutations, and activation of oncogenic pathways (e.g., RAS–mitogen-activated protein kinase [MAPK], AKT–mammalian target of rapamycin [mTOR], and MET [a hepatocyte growth factor receptor]).
笔记:
- 英文中常见的结构出现了很多....此外,be characterized by的处理,也很经典。
与不良临床结局相关的大多数基因标记也在增殖型中富集12 。
Most of the gene signatures associated with a poor clinical outcome are also enriched in the proliferation class.12
非增殖型肿瘤有较多CTNNB1(β-连环蛋白)突变,其基因表达模式与正常肝细胞相似。
Tumors of the nonproliferation class have more CTNNB1 (beta-catenin) mutations, and their gene-expression pattern resembles that of normal hepatocytes.
笔记:
- 很友好的并列句,resemble这个单词比similar啥的,好玩儿多了...
肝细胞癌是包含非肿瘤细胞(主要是免疫相关细胞)的复杂生态系统。
Hepatocellular carcinomas are complex ecosystems incorporating nontumor cells, mainly immune-related cells.
笔记:
- incorporate在线学习...你值得替换include
免疫检查点抑制在实体瘤中取得的成功突显了肿瘤微环境在癌症进展中的关键作用。
The success of immune checkpoint inhibition in solid tumors underscores the key role of the tumor microenvironment in the progression of cancer.
笔记:
- 额外知识点:underscore是英式用法,美式常用underline...
大约30%的早期肝细胞癌有免疫激活的基因组证据13 ,而25%无免疫浸润。
Approximately 30% of early-stage hepatocellular carcinomas have genomic evidence of immune activation,13 whereas 25% have no immune infiltrate.
了解癌细胞与其微环境之间的相互作用对于开发新疗法和识别生物标志物至关重要。
Understanding the interaction between cancer cells and their microenvironment will be crucial for developing new therapies and identifying biomarkers.
危险因素
Risk Factors
肝细胞癌在无肝病的患者中罕见,男性患病率是女性的两倍。
Hepatocellular carcinoma is rare among patients without liver disease and is twice as common in men as in women.
笔记:
- twice as common in A as in B A是B的两倍
任何原因的肝硬化均会增加肝细胞癌的发生风险,肝细胞癌的年发病率为2%~4%。
Cirrhosis of any cause increases the risk of hepatocellular carcinoma, with an annual incidence between 2 and 4%.
笔记:
- A of any cause 任何原因的A
肝细胞癌风险因病因、地区、性别、年龄和肝损伤程度不同而异14 。
This risk varies according to cause, geographic area, sex, age, and degree of liver damage.14
笔记:
- vary
在全球范围内,HBV感染是发生肝细胞癌的主要原因。
Worldwide, HBV infection is the main cause of hepatocellular carcinoma.
笔记:
- Worldwide
虽然接种HBV疫苗降低了肝细胞癌的发病率15 ,但许多未接种疫苗的人仍然感染HBV(2015年为2.57亿),因此有患肝细胞癌的风险,这些人主要在亚洲和撒哈拉以南非洲地区16 。
Although HBV vaccination reduces the incidence of hepatocellular carcinoma,15 many unvaccinated persons are still infected with HBV (257 million in 2015) and thus at risk for hepatocellular carcinoma, mostly in Asia and sub-Saharan Africa.16
笔记:
- and后的are省略,很地道
- unvaccinated 棒
通过饮食暴露于黄曲霉素B1进一步增加了HBV感染者患肝细胞癌的风险,其机制是通过TP53在249位点的特定突变。
Dietary exposure to aflatoxin B1 amplifies the risk of hepatocellular carcinoma among patients with HBV infection, through a specific mutation in TP53 at position 249 (R→S).
笔记:
- dietary exposure to 通过饮食暴露于
- 中文中的“其机制是”为增译
在西方国家和日本,肝细胞癌的主要原因是HCV感染。
In Western countries and Japan, the main cause of hepatocellular carcinoma is HCV infection.
HBV感染具有直接致癌作用,不论基础肝纤维化程度如何17 ,而无晚期纤维化的HCV感染者很少发生肝细胞癌。
HBV infection has a direct oncogenic effect, regardless of the degree of underlying liver fibrosis,17 but hepatocellular carcinoma rarely occurs in HCV-infected patients who do not have advanced fibrosis.
笔记:
- oncogenic effect 致癌作用
全球范围内NAFLD导致的肝细胞癌发病率呈上升趋势。
The incidence of hepatocellular carcinoma due to NAFLD is increasing worldwide.
在美国,预计从2016至2030年,发病率将增加122%,即从5,510例增加至12,240例18 。
In the United States, the incidence is expected to increase by 122% between 2016 and 2030, from 5510 to 12,240 cases.18
酒精性肝硬化是肝细胞癌的另一个常见原因。
Alcoholic cirrhosis is another frequent cause of hepatocellular carcinoma.
吸烟和同时感染人类免疫缺陷病毒也可促进肝细胞癌的发生。
Smoking and coinfection with the human immunodeficiency virus can also contribute to the development of hepatocellular carcinoma.
笔记:
- the development of A处理为了A的发生,存疑。
- contribute to 促进
抗病毒治疗可有效降低肝细胞癌的发病率,但不能消除风险19 。
Antiviral therapies are effective in reducing the incidence of hepatocellular carcinoma but do not eradicate the risk.19
笔记:
- be effective in v-ing 有效做某事
- eradicate 这个词很有意思
与干扰素无效的HCV感染者相比,在有持续病毒学应答的感染者中,肝细胞癌风险从6.2%降至1.5%20 。
Among patients with HCV infection who have a sustained virologic response to interferon-based treatment regimens, the risk of hepatocellular carcinoma is reduced from 6.2 to 1.5%, as compared with patients who do not have a response.20
笔记:
- interferon 干扰素
- a sustained virologic response to 持续病毒学应答
- 中英文句子之间的结构差异,值得细细品味
据报道,接受直接抗病毒药治疗的HCV感染者的肝细胞癌风险降低21 。
Reduction in the risk of hepatocellular carcinoma has been reported among HCV-infected patients treated with direct-acting antiviral agents.21
笔记:
- 英文行文的常见差异。英文主语为全句强调重点。
这些患者通常比接受干扰素治疗的患者有更严重的纤维化22 。
Such patients typically have more advanced fibrosis than those who received interferon-based therapies.22
除了治疗肝病的病因之外,目前尚无已知可降低肝细胞癌发病率的药物。
Apart from treatment of the cause of the liver disease, no drugs are known to reduce the incidence of hepatocellular carcinoma.
监测
Surveillance
癌症监测旨在早期发现肿瘤,增加根治机会,提高生存率。
Cancer surveillance aims to detect tumors at early stages, increase the opportunity to use curative treatments, and improve survival.
笔记:
- 英文为三个并列结构,值得注意
然而,尚无高质量的随机对照试验评估肝硬化患者的肝细胞癌监测效果。
However, no high-quality randomized, controlled trials have evaluated the effect of hepatocellular carcinoma surveillance in patients with cirrhosis.
笔记:
- 未用there be的句型
一项研究突显了开展此类试验的难度,因为99%的患者拒绝承担被随机分配到非监测组后面临的风险23 。
One study underscored the difficulties of conducting such a trial, with 99% of patients declining to assume the risk of being randomly assigned to the nonsurveillance group.23
笔记:
- underscore的美式变形,还记得么?
- with + v-ing的结构
- 这一句也是常见的英文行文:主句表结论,with后面的附属结构陈述具体细节
尽管如此,数学模型24 、低质量的临床试验(有方法学局限性)25 以及更重要的是队列研究26 的荟萃分析均显示肝癌监测具有生存获益。
Nonetheless, mathematical models,24 a low-quality clinical trial (with methodologic limitations),25 and more important, a meta-analysis of cohort studies26 all show survival benefits of surveillance.
笔记:
- 这一句除了插入语,没啥细节可说的
这一证据支持各个重要肝病专业学会提出的监测建议27-29 。
This evidence substantiates the recommendation of surveillance by major hepatology professional societies.27-29
肝癌监测最近面临的批评包括非随机研究表明监测的净获益小,而假阳性结果造成的危害增加30 ,以及疾病特异性死亡率并无降低31 。
Recent criticisms of surveillance include a small net benefit with increased harms as a result of false positive results30 and lack of a decrease in disease-specific mortality,31 on the basis of nonrandomized studies.
笔记:
- 中文和之前有一句的译文很像,初看以为是三者的并列结构。但在英文表达中可以发现,作者最希望强调的是a small net benefit,with后面的部分不过是其他附属的信息。
监测的目标人群是肝硬化患者,无论其病因如何。
The target population for surveillance is patients with cirrhosis, regardless of the cause.
笔记:
- 这句翻译,翻译腔,或者说机器翻译痕迹有些明显了。
肝硬化患者中肝细胞癌的年发病率超过1.5%,而这个是使监测符合成本效益的阈值32 。
The annual incidence of hepatocellular carcinoma among these patients surpasses the threshold of 1.5% that renders surveillance cost-effective.32
笔记:
- 这句翻译的质量也不如预期,或许是交稿时间太紧了吧?强行口译的顺句驱动技巧,在笔译稿子里看,真是拗口无比。
肝硬化合并晚期肝功能障碍的患者无法接受根治,因此不是监测的目标人群。
Patients with cirrhosis and advanced liver dysfunction, who would not be eligible for a curative treatment, are not candidates for surveillance.
笔记:
- 这句明显质量有回升,句子间的逻辑处理也很经典
这个不适用于因肝功能障碍而适合肝移植的患者。
This does not apply to candidates for liver transplantation as a result of liver dysfunction.
笔记:
- 这个?译者....this原文未明确,译者或应表述清楚。个人认为,这一句参考上文语境,或为“该定义···” 直接用“这个”,有点不走心。
对移植等候名单上的患者进行监测的目的是确保患者未发生根据既定标准使其不再适合肝移植的肿瘤。
The goal of surveillance for patients on the transplant waiting list is to ensure that a tumor does not develop that would preclude transplantation according to established criteria.
笔记:
- 这一句的中文翻译,对读者的肺活量提出了较高的要求。
一些无肝硬化的肝病患者也应纳入监测项目。
Some patients who have liver disease without cirrhosis should also be enrolled in surveillance programs.
慢性HBV感染者的肝细胞癌风险因地区不同而异(非洲和亚洲的风险高于其他地区),并因以下因素而增加:年龄增长、男性、肝纤维化、高水平病毒复制、基因型C和肝细胞癌家族史33 。
The risk of hepatocellular carcinoma among patients with chronic HBV infection varies across geographic regions (with higher risks in Africa and Asia than in other regions) and increases with age, male sex, presence of liver fibrosis, high level of viral replication, genotype C, and a family history of hepatocellular carcinoma.33
笔记:
- 看似很长,但逻辑很简单的并列句
目前有多个用于量化风险的评分系统,但均未得到广泛认可,原因是跨地区的验证结果欠佳34 。
Various scoring systems are available to quantify this risk, but none are universally accepted because of suboptimal validation across geographic regions.34
笔记:
- suboptimal 很有意思的一个词
慢性HCV感染合并晚期纤维化的患者(Metavir系统将其定义为F3或以上,Metavir系统的评分范围是F0~F4,评分较高表示纤维化较严重,参见补充附录表S1,补充附录与本文原文可在NEJM.org获取)35 面临的风险足够高,应进行监测。
Patients with chronic HCV infection and advanced fibrosis, defined by the Metavir system as a score of F3 or higher on a scale of F0 to F4, with higher scores indicating more severe fibrosis (see Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org),35 are at sufficient risk to undergo surveillance.
笔记:
- at sufficient risk to do sth. 英文经典,翻译也很好
虽然肝细胞癌可能发生于无肝硬化的NAFLD患者,但实际风险未知,而且很可能非常低。
Although hepatocellular carcinoma can arise in patients who have NAFLD without cirrhosis, the actual risk is unknown and probably very low.
在开发出识别风险增加患者的方法之前,不建议对无肝硬化的NAFLD患者进行监测。
Until there are methods available to identify patients who are at increased risk, surveillance is not recommended for patients who have NAFLD without cirrhosis.
推荐采用的监测方法是每6个月进行1次腹部超声检查,同时测定或不测定血清甲胎蛋白水平。
Abdominal ultrasonography every 6 months is the recommended method for surveillance, with or without measurement of serum levels of alpha-fetoprotein.
笔记:
- with or without 很值得学习
检查结果受操作者影响,其灵敏度为47%~84%,特异度高于90%36 。
The results are operator-dependent, with a sensitivity of 47 to 84% and a specificity higher than 90%.36
超声检查对肥胖患者的监测性能存在问题。
The performance of ultrasound surveillance is problematic in obese patients.
笔记:
- problematic in
计算机断层扫描(CT)或磁共振成像(MRI)作为监测工具的作用尚不清楚。
The role of computed tomography (CT) or magnetic resonance imaging (MRI) as a surveillance tool is unknown.
液体活检中的循环肿瘤DNA突变分析已成为早期检测肝癌的潜在新工具37 ,但其性能尚未确定。
Mutation analysis of circulating tumor DNA in the context of liquid biopsy has emerged as a potential new tool for early detection,37 but its performance has yet to be established.
诊断
Diagnosis
在肝硬化患者中,肝细胞癌可通过影像学技术诊断(图2),因为肝细胞恶变过程中发生血管转变,其中良性病变(例如再生结节和发育不良结节)由门静脉系统分支供血,而恶性结节由肝动脉供血38 。
In patients with cirrhosis, hepatocellular carcinoma can be diagnosed with the use of imaging techniques (Figure 3) because of the vascular shift that occurs during malignant transformation of hepatocytes, in which benign lesions (e.g., regenerative and dysplastic nodules) are supplied by branches of the portal system, whereas malignant nodules are supplied by blood from the hepatic artery.38
笔记:
- cirrhosis 口译时需注意发音
- imaging techniques 影像学技术
- 再见顺句驱动翻译,是否可以考虑先因后果的处理,似乎确要通顺些。
上述转变将转化为CT或MRI增强扫描中的独特模式,即动脉相超增强,静脉相或延迟相洗脱。
This shift translates into a distinctive pattern of hyperenhancement in the arterial phase and washout in venous or delayed phases on contrast-enhanced CT or MRI.
笔记:
- washout 洗脱
对于结节直径大于1 cm的肝硬化患者,上述模式诊断肝细胞癌的灵敏度为66%~82%,特异度高于90%39 。
This pattern has a sensitivity between 66% and 82% and a specificity higher than 90% for the diagnosis of hepatocellular carcinoma in patients with cirrhosis and nodules larger than 1 cm in diameter.39
笔记:
specificity 特异度
肝脏影像学报告和数据系统(Liver Imaging Reporting and Data System)利用这些和其他特征,根据肝脏结节是肝细胞癌的可能性对其进行了分类40 。
The Liver Imaging Reporting and Data System uses these and other features to classify hepatic nodules on the basis of the likelihood that they represent hepatocellular carcinoma.40
主要位于欧洲27 和亚洲28 的专家中心也运用超声造影检查了确定肝脏结节的特征,但其精确的诊断作用仍在研究中。
Contrast-enhanced ultrasonography is also used to characterize liver nodules at expert centers, mostly in Europe27 and Asia,28 but its precise diagnostic role is still under investigation.
对于影像学检查中模式不明确的结节或无肝硬化患者的结节,疾病诊断有赖于活检。
For nodules with an inconclusive pattern on imaging or those in patients without cirrhosis, the diagnosis should rely on biopsy.
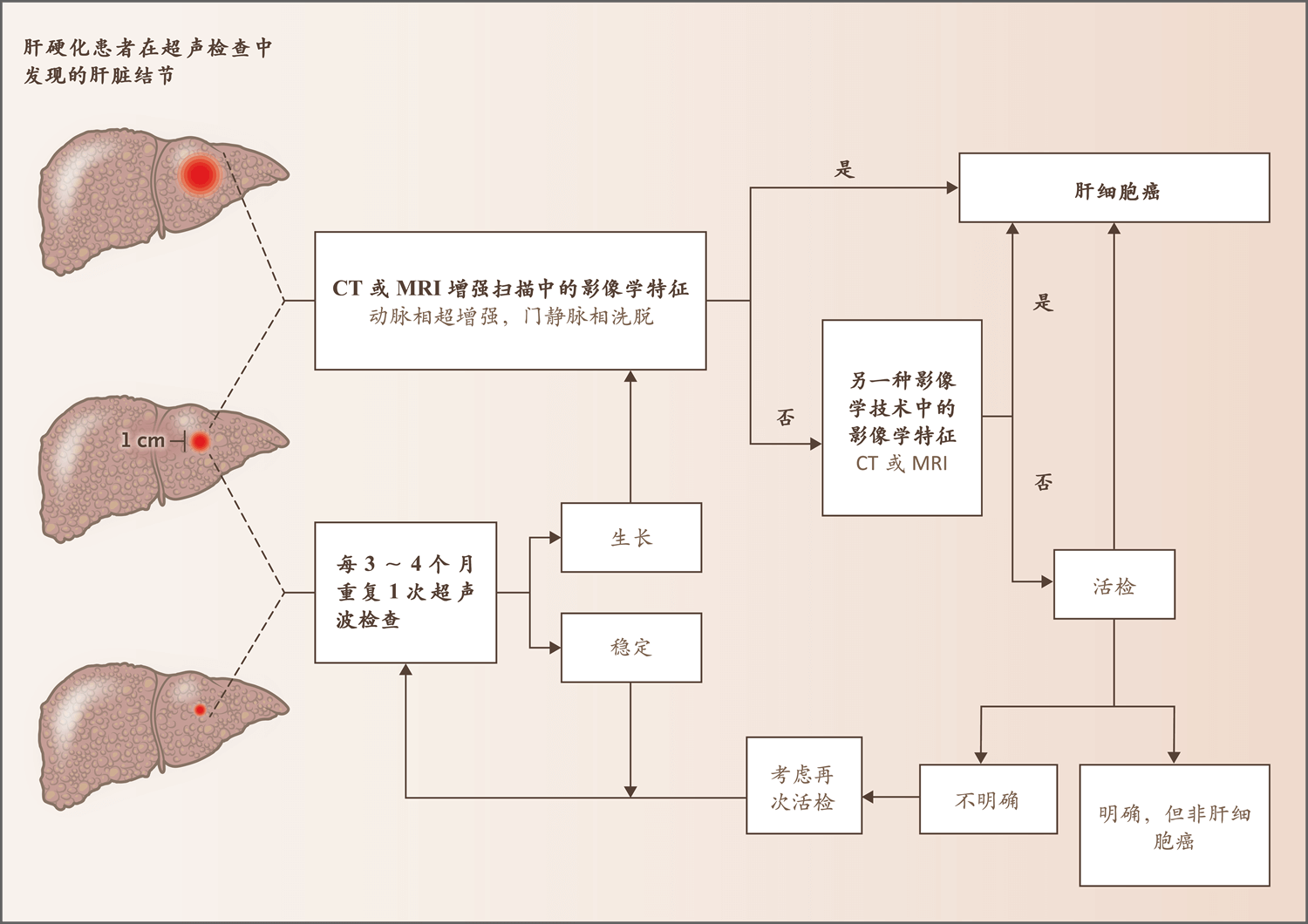
图2. 肝硬化患者肝脏结节的诊断流程
Figure2. Diagnostic Algorithm for a Liver Nodule in a Patient with Cirrhosis.
该流程改编自欧洲肝脏研究学会(European Association for the Study of the Liver)指南27 。
The algorithm has been adapted from the guidelines of the European Association for the Study of the Liver.27
肝硬化患者的肝细胞癌可采用影像学技术,根据结节大小和影像学模式进行诊断。
Hepatocellular carcinoma in patients with cirrhosis can be diagnosed with the use of imaging techniques, depending on the size of the nodule and the pattern on radiologic imaging.
如果直径小于1 cm的结节在12个月后大小保持稳定,则应考虑恢复6个月1次的常规监测。
If a nodule less than 1 cm in diameter remains stable in size after 12 months, a return to regular 6-month surveillance should be considered.
笔记:
- remian stable in size
- a return to
美国肝病研究学会(American Association for the Study of Liver Diseases)指南建议,对于不明确的结节,随访影像学检查可作为活检的可能的替代方法29 。
The guidelines of the American Association for the Study of Liver Diseases recommend follow-up imaging as a possible alternative to biopsy for indeterminate nodules.29
笔记:
- indeterminate
为有小结节的患者建立组织学诊断可能具有挑战性,但一组免疫染色标志物(磷脂酰肌醇蛋白聚糖-3、热休克蛋白70和谷氨酰胺合成酶)提高了诊断的准确度41 。
Establishing a histologic diagnosis in a patient with small nodules can be challenging, but a set of immunostaining markers — glypigan 3, heat shock protein 70, and glutamine synthetase — increase diagnostic accuracy.41
结节直径小于1 cm的肝硬化患者应每3~4个月进行1次超声监测,如果结节大小在12个月后保持稳定,可考虑恢复常规监测27 。
Patients who have cirrhosis with nodules that are less than 1 cm in diameter should undergo ultrasound surveillance every 3 to 4 months and be considered for a return to conventional surveillance if the nodule is stable in size after 12 months.27
分期
Staging
由于大多数肝细胞癌患者合并肝病,因此对于肝硬化患者,必须权衡肿瘤治疗的获益和药物干预的潜在危害。
Since most patients with hepatocellular carcinoma have concomitant liver disease, the benefits of treating the tumor must be balanced against the potential harms of medical interventions in patients with cirrhosis.
笔记:
- concomitant liver disease 合并肝病(concomitant的发音的重音也需要注意)
- be balanced against 权衡
肝细胞癌治疗中的复杂性要求我们采用多学科方法,结合肝病学、肝胆外科、病理学、肿瘤学、放射学(诊断和干预)和专科护理等方面的专业技能。
This complexity in the management of hepatocellular carcinoma calls for a multidisciplinary approach, with expertise in hepatology, hepatobiliary surgery, pathology, oncology, radiology (both diagnostic and interventional), and specialized nursing.
笔记:
- a multidisciplinary approach
- call for
为了正确估计生存期,分期系统不仅必须量化肿瘤负担,还必须量化肝功能障碍的程度和患者的体能状态。
To adequately estimate survival, any staging system must quantify not only the tumor burden but also the extent of liver dysfunction and performance status.
1999年提出的肝癌巴塞罗那(BCLC)分期对上述各部分进行衡量42 ,是目前应用最广泛的分期系统43 。
All these components are measured in the Barcelona Clinic Liver Cancer (BCLC) algorithm, which was introduced in 1999 and is the staging system most widely applied.
该分期获得临床实践指南27,29 的支持,并且是肝细胞癌临床试验设计的公认基准27 。
This algorithm is endorsed in clinical practice guidelines27,29 and is the accepted benchmark for clinical trial design in hepatocellular carcinoma.27
该分期将患者分成五期,并为每个分期提出治疗建议(图3)。
The algorithm classifies patients as being in one of five stages and provides treatment recommendations for each stage (Figure 3).
除此之外还有其他分期系统(例如香港肝癌分期系统[Hong Kong Liver Cancer staging system]44 和意大利肝癌项目[Cancer of the Liver Italian Program]45 ),但它们的应用仅限于某些地区。
Other staging systems exist (e.g., the Hong Kong Liver Cancer staging system44 and the Cancer of the Liver Italian Program45), but their implementation is restricted to certain geographic areas.
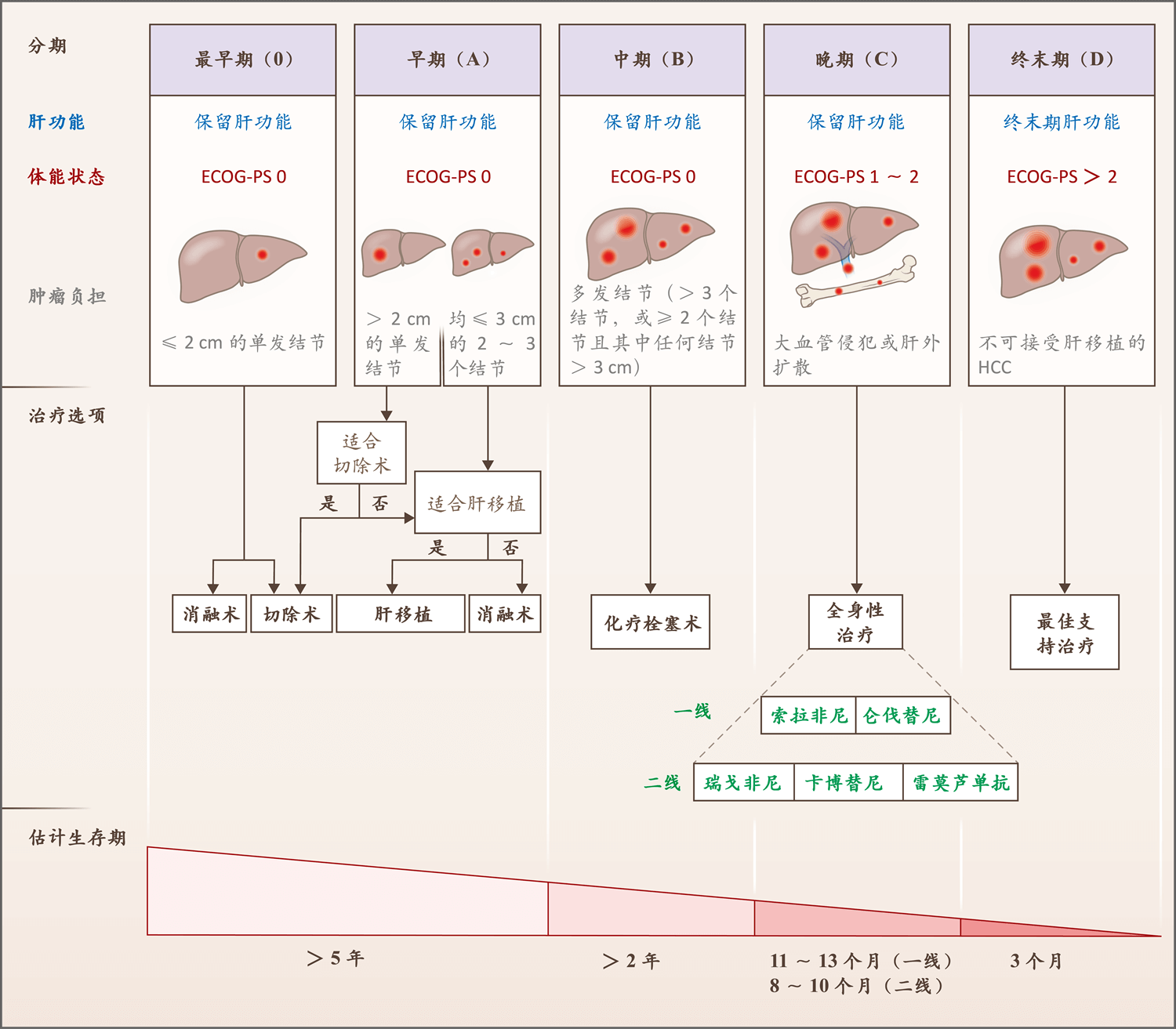
图3.肝细胞癌治疗的临床流程
Figure 3. Clinical Algorithm for the Management of Hepatocellular Carcinoma.
上述流程是基于肝癌巴塞罗那分期(该分期将患者分成五期)和欧洲肝脏研究学会指南27,43 。
The algorithm is based on the Barcelona Clinic Liver Cancer algorithm, which classifies patients as being in one of five stages, and European Association for the Study of the Liver guidelines.27,43
图中绘制的是采用推荐治疗的情况下,每个分期的估计生存期。
Depicted is the estimated survival time in each stage once the recommended therapy has been administered or performed.
仑伐替尼试验未纳入肿瘤的肝占位≥50%的患者,也未纳入发生胆管或门静脉主干侵犯的患者。
The lenvatinib trial did not include patients with 50% or higher occupation of the liver with tumors or invasion of the bile duct or main portal vein.
二线治疗均未在接受过仑伐替尼治疗的患者中进行试验。
None of the second-line therapies were tested in patients who had tumors that had been treated with lenvatinib.
瑞戈非尼在索拉非尼副作用可接受的患者中进行了试验,雷莫芦单抗在血清AFP水平≥400 ng/mL的患者中进行了试验。
Regorafenib was tested in patients for whom sorafenib was associated with an acceptable side-effects profile, and ramucirumab in those with serum AFP levels of 400 ng per milliliter or greater.
美国东部肿瘤协作组体能状态(ECOG-PS)是一个五分量表,较高评分反映较严重失能。
Eastern Cooperative Oncology Group performance status (ECOG-PS) is a five-point scale on which higher numbers reflect greater disability.
HCC表示肝细胞癌。
HCC denotes hepatocellular carcinoma.
肿瘤负荷利用横断面影像学检查,根据结节数量和大小以及是否有大血管侵犯或肝外扩散进行量化。
The tumor burden is quantified according to the number and size of nodules, along with the presence or absence of macrovascular tumor invasion or extrahepatic spread, as assessed with cross-sectional imaging.
笔记:
- the presence or absence of
对于肝功能障碍评估,传统的Child–Turcotte–Pugh评分(补充附录表S2)46 提供了主观评估结果,但未能充分反映肝功能储备。
For the assessment of liver dysfunction, the traditional Child–Turcotte–Pugh score (Table S2 in the Supplementary Appendix)46 provides a subjective assessment and does not adequately capture the hepatic functional reserve.
替代方案包括终末期肝病模型(Model for End-Stage Liver Disease)47 和白蛋白-胆红素分级48 。
Alternatives include the Model for End-Stage Liver Disease47 and the albumin–bilirubin grade.48
这些评估方法均有局限性,但区分保留肝功能的患者和较晚期肝病患者至关重要。
All these assessment methods have limitations, but it is crucial to distinguish patients with well-preserved liver function from those with more advanced hepatopathy.
在临床实践中,保留肝功能的患者指的是患代偿性肝病(即无腹水)49 ,并且Child–Turcotte–Pugh评分为A(范围,A~C,C比A的肝功能障碍严重)的患者。
In practical terms, patients with well-preserved liver function are those with compensated disease (i.e., no ascites)49 and a Child–Turcotte–Pugh score of A on a scale of A to C, with C indicating more liver dysfunction than A.
这些患者在疾病各个分期的结局均最好。
These patients have the best outcomes across disease stages.
患者的总体健康状况,以及在无须他人帮助的情况下完成某些日常生活活动的能力通过美国东部肿瘤协作组体能状态进行衡量,这是一个五分量表,较高评分反映较严重失能50 。
A patient’s general well-being and ability to perform certain activities of daily living without the help of others are measured with the Eastern Cooperative Oncology Group performance status, a five-point scale on which higher numbers reflect greater disability.50
临床治疗
Clinical Management
外科治疗
SURGICAL THERAPIES
最适合切除术的是有早期(BCLC分期0或A)单发肿瘤(不论肿瘤大小如何)、体能状态良好、肝功能保留良好并且无临床显著门静脉高压的患者51 。
The ideal candidates for resection are patients with a solitary tumor at an early stage (BCLC stage 0 or A), regardless of tumor size, in whom the performance status is good, liver function is well preserved, and there is no clinically significant portal hypertension.51
这些患者接受切除术后的5年生存率超过60%,且术后死亡率低(<3%);5年时多达70%的患者有肿瘤复发52 。
For these patients, resection is associated with survival above 60% at 5 years, with low postoperative mortality (<3%); as many as 70% of these patients have tumor recurrence at 5 years.52
尚无任何经证明可减少复发的辅助治疗53 。
No adjuvant therapies have been shown to reduce recurrence.53
在接受切除术的患者中,尽管基因标记的应用改善了预后分层12 ,但该方法尚未成为常规临床实践的一部分。
Despite improved prognostic stratification with the use of gene signatures in patients who have undergone resection,12 this approach has not become part of routine clinical practice.
笔记:
- with the use of
近期一项非对照研究纳入的是患早期肿瘤,并且正在接受直接抗病毒药的HCV感染者,结果表明切除术或消融术后肿瘤复发的患者比例出乎意料地高54 。
A recent uncontrolled study involving HCV-infected patients with early-stage tumors who were receiving direct-acting antiviral agents showed an unexpectedly high percentage of patients with tumor recurrence after resection or ablation.54
笔记:
- 英文原句的逻辑层次很清晰,很舒服
之后的研究55 (包括荟萃分析)未能证实这些结果56 ,因此我们需要更多数据来确定这些药物是否有利于肿瘤复发。
Subsequent studies,55 including a meta-analysis, failed to confirm these results,56 so additional data are needed to determine whether these drugs favor tumor recurrence.
笔记:
- 个人觉得,应该为“包括一次荟萃分析”,这个冠词似乎有点用处
- 后半句的增译“我们”,是否需要加?也存疑。或可处理为“这些药物是否有利于肿瘤复发,还需更多数据来确定。”
肿瘤负荷有限但不适合切除术的患者可进行肝移植。
Liver transplantation can be performed in patients with a limited tumor burden who are not candidates for resection.
笔记:
- 英文与中文的主语选择值得留意。
- tumor burden
- candidate for sth. 适合做sth.
肝移植除移除肿瘤之外,还具有根治肝病的优点。
In addition to removing the tumor, transplantation has the advantage of curing the liver disease.
肝移植米兰标准(即直径≤5 cm的单发结节,或者直径均≤3 cm的最多3个结节)是肝细胞癌患者的基准57 ,已被器官共享联合网络(UNOS)采纳。
The Milan criteria for liver transplantation (i.e., a single nodule ≤5 cm in diameter or up to three nodules, none larger than 3 cm in diameter) are the benchmark in patients with hepatocellular carcinoma57 and have been adopted by the United Network for Organ Sharing (UNOS).
肿瘤侵犯大血管或肝外扩散是肝移植的禁忌证,因为肿瘤复发的风险高。
Macrovascular tumor invasion or extrahepatic spread is a contraindication for transplantation because of the high risk of tumor recurrence.
笔记:
- Macrovascular tumor invasion or extrahepatic spread 肿瘤侵犯大血管或肝外扩散
符合米兰标准的肿瘤患者接受肝移植后,5年生存率为60%~80%,10年生存率为50%,移植后肿瘤复发率低于15%。
Transplantation in patients with tumors that meet the Milan criteria is associated with survival of 60 to 80% at 5 years and 50% at 10 years, with post-transplantation tumor recurrence lower than 15%.
笔记:
- be associated with 这个处理可以规避一些老套的句式
不符合米兰标准的肿瘤患者接受肝移植后结局较差58 。
Transplantation in patients with tumors that do not meet the Milan criteria has worse outcomes.58
对等候名单上的患者采取新辅助治疗(一般是消融术或经动脉治疗)是符合成本效益的方法,在中位等候时间超过6个月的情况下可减少肿瘤进展导致的脱落人数59 。
Neoadjuvant treatments administered while patients are on the waiting list, generally ablation or transarterial therapies, are a cost-effective means of reducing the number of dropouts due to tumor progression when the median waiting time is longer than 6 months.59
笔记:
- 译文断句很棒
UNOS允许通过这些治疗方法将超过米兰标准的某些肿瘤降低分期(即通过降低肿瘤负荷的方式达到米兰标准),从而使之前不适合肝移植的患者适合肝移植。
The use of these therapies to down-stage some tumors exceeding the Milan criteria (i.e., reducing the tumor burden to meet the criteria) is accepted by UNOS as a way of making formerly ineligible patients eligible for transplantation.
笔记:
- down-stage用成动词
边缘供肝和活体捐献扩大了可供移植的器官库。
The use of marginal grafts and living donation has increased the pool of organs available for transplantation.
然而,供者和等待名单上患者之间的人数差距仍然是肝移植的主要限制因素。
However, the gap between available donors and patients on the waiting list is still a major limitation of transplantation.
肿瘤消融术
TUMOR ABLATION
不适合手术的0或A期肿瘤患者被推荐接受消融术27,29 。
Ablation is recommended in patients with BCLC stage 0 or A tumors who are not candidates for surgery.27,29
笔记:
- 这个in是重点
- 适合/不适合,英语表达法
主要方法是影像引导的经皮射频消融术,通过诱导肿瘤内高温的方式实现肿瘤坏死。
The main method is image-guided, percutaneous radiofrequency ablation, which achieves tumor necrosis by inducing a high intratumoral temperature.
笔记:
- 这一句做视译很巴适
肿瘤坏死程度与肿瘤大小呈负相关,肿瘤直径大于3 cm的情况下,坏死程度显著下降。
The extent of tumor necrosis is negatively correlated with tumor size and drops significantly in tumors larger than 3 cm in diameter.
笔记:
- 正相关/负相关的表达很经典
与切除术相比,消融术并发症较少,但对较大肿瘤的局部控制效果较差。
As compared with resection, ablation has fewer complications but provides worse local control for larger tumors.
笔记:
- compare与than不同时使用,是经典易错点
对于有直径小于2 cm的单发肿瘤,且位于肝实质内有利位置的某些患者,射频消融术与切除术均可作为推荐的一线治疗60 。
In some patients with solitary tumors that are less than 2 cm in diameter and in a favorable location within the liver parenchyma, radiofrequency ablation competes with resection as a recommended option for frontline treatment.60
笔记:
- solitary tumor 单发肿瘤
- liver parenchyma 肝实质
- compete with sth. for sth.这个结构的译文处理有点意思
其他消融术包括微波消融、冷冻消融和乙醇注射。
Other ablative options include microwave ablation, cryoablation, and ethanol injection.
外照射放疗是安全的,但需要随机对照试验来确定其在肝细胞癌治疗中的疗效和作用。
External-beam radiotherapy is safe, but randomized, controlled trials are needed to determine its efficacy and role in the management of hepatocellular carcinoma.
经动脉治疗
TRANSARTERIAL THERAPIES
中期肿瘤(BCLC B期)患者应考虑经动脉治疗。
Patients with intermediate-stage tumors (BCLC stage B) should be considered for transarterial therapies.
主要的治疗方法是经动脉化疗栓塞术(TACE),该方法需要在动脉内输入细胞毒性药物,然后立即栓塞肿瘤的供血血管。
The main treatment method is transarterial chemoembolization (TACE), which entails intraarterial infusion of a cytotoxic agent, followed immediately by embolization of the vessels that feed the tumor.
笔记:
- 金山词霸里的发音水平存疑,建议学好音标...比如chemoembolization,金山词霸发音目前是错的(2019年4月)。
- entail,日常可以替代谁?
邻近的非肿瘤性肝组织通常不受TACE的影响,因为与肿瘤不同,其供血主要来自门静脉。
Adjacent nontumoral liver tissue is generally protected from TACE because, unlike the tumor, its blood supply comes mainly from the portal vein.
笔记:
- portal vein: a vein that takes blood from the stomach and other organs near the stomach to the live
两项随机对照试验以及一项包括阳性和阴性试验的荟萃分析显示,TACE与最佳支持治疗相比具有生存获益61 。
Two randomized, controlled trials and a meta-analysis that included positive and negative trials showed survival benefits with TACE as compared with the best supportive care.61
笔记:
- the best supportive care 最佳支持治疗
包括101项研究,12,372例患者的一项TACE系统综述表明客观缓解率为52.5%62 。
A systematic review of TACE, including 101 studies and 12,372 patients, showed an objective response of 52.5%.62
与TACE相关的死亡率低于1%,大多数死亡原因是肝衰竭62 ,这一发现强调指出肝癌治疗中正确选择患者的重要性。
The mortality associated with TACE was below 1%, with most deaths due to liver failure,62 a finding that underscores the importance of adequate patient selection for this therapy.
笔记:
- a finding that
- 在表强调时,underscore已经出现多次
- adequate
失代偿期肝硬化患者不应考虑TACE。
TACE should not be considered in patients with decompensated cirrhosis.
有证据表明,使用药物洗脱珠进行的TACE具有与常规TACE相似的抗肿瘤活性,且副作用较少,而非载药栓塞(bland栓塞)的应用有较大争议。
There is evidence that TACE performed with the use of drug-eluting beads has antitumoral activity similar to that of conventional TACE, with fewer side effects, whereas the use of bland embolization is more controversial.
笔记:
- drug-eluting beads 药物洗脱珠
- whereas
TACE的中位生存期为26~40个月,具体取决于患者选择是否适当63,64 。
Median survival with TACE ranges from 26 to 40 months, depending on patient selection.63,64
TACE联合索拉非尼(丝氨酸-苏氨酸激酶Raf-1和B-Raf,以及血管内皮生长因子受体[VEGFR]和血小板源性生长因子受体-β[PDGFR-β]的受体酪氨酸激酶活性抑制剂)或布立尼布(brivanib,VEGFR和成纤维细胞生长因子受体抑制剂)全身性治疗未改善患者生存63,65 。
Combining TACE with the systemic drug sorafenib (an inhibitor of the serine–threonine kinases Raf-1 and B-Raf and the receptor tyrosine kinase activity of vascular endothelial growth factor receptors [VEGFRs] and platelet-derived growth factor receptor β [PDGFR-β]) or brivanib (an inhibitor of VEGFR and fibroblast growth factor receptor) does not improve survival.63,65
选择性体内放疗(SIRT)是另一种经动脉治疗方法,常用于BCLC B期肿瘤患者。
Selective internal radiation therapy (SIRT) is another transarterial treatment approach frequently used in patients with BCLC stage B tumors.
其方法是动脉内输入放射性同位素钇-90微球。
It is based on the intraarterial infusion of microspheres with the radioisotope yttrium-90.
与TACE不同,SIRT不包括肉眼可见栓塞步骤(macroembolic step)。
Unlike TACE, SIRT does not include a macroembolic step.
笔记:
- unlike出现频率比differ from高些呢
钇-90产生的辐射是其抗肿瘤活性的机制。
The radiation emitted by yttrium-90 is responsible for its antitumoral activity.
笔记:
- 需要医学背景确认,be responsible for 处理为 ...的机制
尚无随机3期临床试验比较接受TACE和SIRT治疗患者的生存期,但大量队列和回顾性研究表明SIRT是一种安全的方法,其客观缓解率与TACE相似66 。
No randomized phase 3 trials have compared TACE and SIRT with respect to survival, but numerous cohort and retrospective studies indicate that SIRT is a safe procedure with an objective response similar to that seen with TACE.66
在晚期疾病(即BCLC C期)患者中评估SIRT的3期临床试验表明,SIRT与索拉非尼相比患者生存无改善,并且SIRT联合索拉非尼与索拉非尼单药治疗相比患者生存无改善67-69 。
The phase 3 clinical trials evaluating SIRT in patients with advanced disease (i.e., BCLC stage C) showed no improvement in survival with SIRT as compared with sorafenib and no improvement with SIRT and sorafenib combined as compared with sorafenib alone.67-69
全身性治疗
SYSTEMIC THERAPIES
全身性治疗推荐用于晚期疾病(BCLC C期)患者或经动脉治疗后发生进展的中期疾病(BCLC B期)患者。 Systemic therapies are recommended for patients who have advanced disease (BCLC stage C) or who have intermediate-stage disease (BCLC stage B) and progression with transarterial therapies.
在关键评估索拉非尼治疗肝细胞癌的随机方案(SHARP)试验中,安慰组患者的生存期为7.9个月,索拉非尼组延长至10.7个月70 。
In the pivotal Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial, survival increased from a median of 7.9 months with placebo to 10.7 months with sorafenib.70
该药物的安全性和中等疗效在亚太地区患者中得到验证71 。
The safety and modest efficacy of this agent were validated in patients from the Asia–Pacific region.71
笔记:
- 冠词不能省略
索拉非尼是美国食品药品管理局(FDA)批准的肝细胞癌第一种全身性治疗药物,也是其标准一线治疗。
Sorafenib was the first systemic drug approved by the Food and Drug Administration (FDA) for the treatment of hepatocellular carcinoma and is the standard of care for frontline therapy.
笔记:
- 处理成并列句
之后的3期试验评估过多种药物和其他治疗方法,其中大部分作为一线治疗未能获得优于或类似于索拉非尼的疗效;而且作为二线治疗未能达到超过安慰剂的患者生存期。
Most agents and other treatment approaches subsequently tested in phase 3 trials failed to improve on or parallel the efficacy of sorafenib as frontline treatment; they also did not increase survival, as compared with placebo, for second-line treatment.
上述药物和治疗方法包括厄洛替尼72 、布立尼布73,74 、舒尼替尼75 、linifanib76 、依维莫司77 、聚乙二醇化精氨酸脱亚胺酶(ADI-PEG20)78 、SIRT67-69 、肝动脉灌注化疗79 、多柔比星80,81 和FOLFOX(氟尿嘧啶、亚叶酸钙[亚叶酸]和奥沙利铂)82 ,以及用于MET过表达患者的tivantinib83 (图4)。
These agents and treatments include erlotinib,72 brivanib,73,74 sunitinib,75 linifanib,76 everolimus,77 pegylated arginine deiminase (ADI-PEG20),78 SIRT,67-69 hepatic arterial infusion chemotherapy,79 doxorubicin,80,81 and FOLFOX (fluorouracil, leucovorin [folinic acid], and oxaliplatin),82 as well as tivantinib in patients with overexpression of MET83 (Figure 4).
笔记:
- 看似复杂,掌控全局更为重要
抗肿瘤活性不足、肝硬化背景下的毒性和患者选择不当被认为是上述失败的原因。
Insufficient antitumoral activity, toxicity in the context of cirrhosis, and inadequate patient selection have been proposed as reasons for these failures.
之前索拉非尼一直是唯一有效的一线治疗方案,直至仑伐替尼(另一种多激酶抑制剂)在非劣效性试验中显示出抗肿瘤活性84 。
Sorafenib remained the sole effective option for frontline therapy until lenvatinib, another inhibitor of multiple kinases, showed antitumoral activity in a noninferiority trial.84
仑伐替尼组的中位生存期为13.6个月,索拉非尼组为12.3个月。
Median survival was 13.6 months with lenvatinib and 12.3 months with sorafenib.
笔记:
- median survival was xxx with DRUG-A
仑伐替尼组的3或4级不良事件包括高血压(仑伐替尼 vs. 索拉非尼,23% vs. 14%)、体重减轻(8% vs. 3%)和掌跖红肿疼痛(3% vs. 11%)。
Grade 3 or 4 adverse events with lenvatinib included hypertension (in 23% of patients, vs. 14% receiving sorafenib), decreased weight (8% vs. 3%), and palmar–plantar erythrodysesthesia (3% vs. 11%).
FDA 2018年批准仑伐替尼用于治疗肝细胞癌。
Lenvatinib received FDA approval for the treatment of hepatocellular carcinoma in 2018.
笔记:
- 主语选择
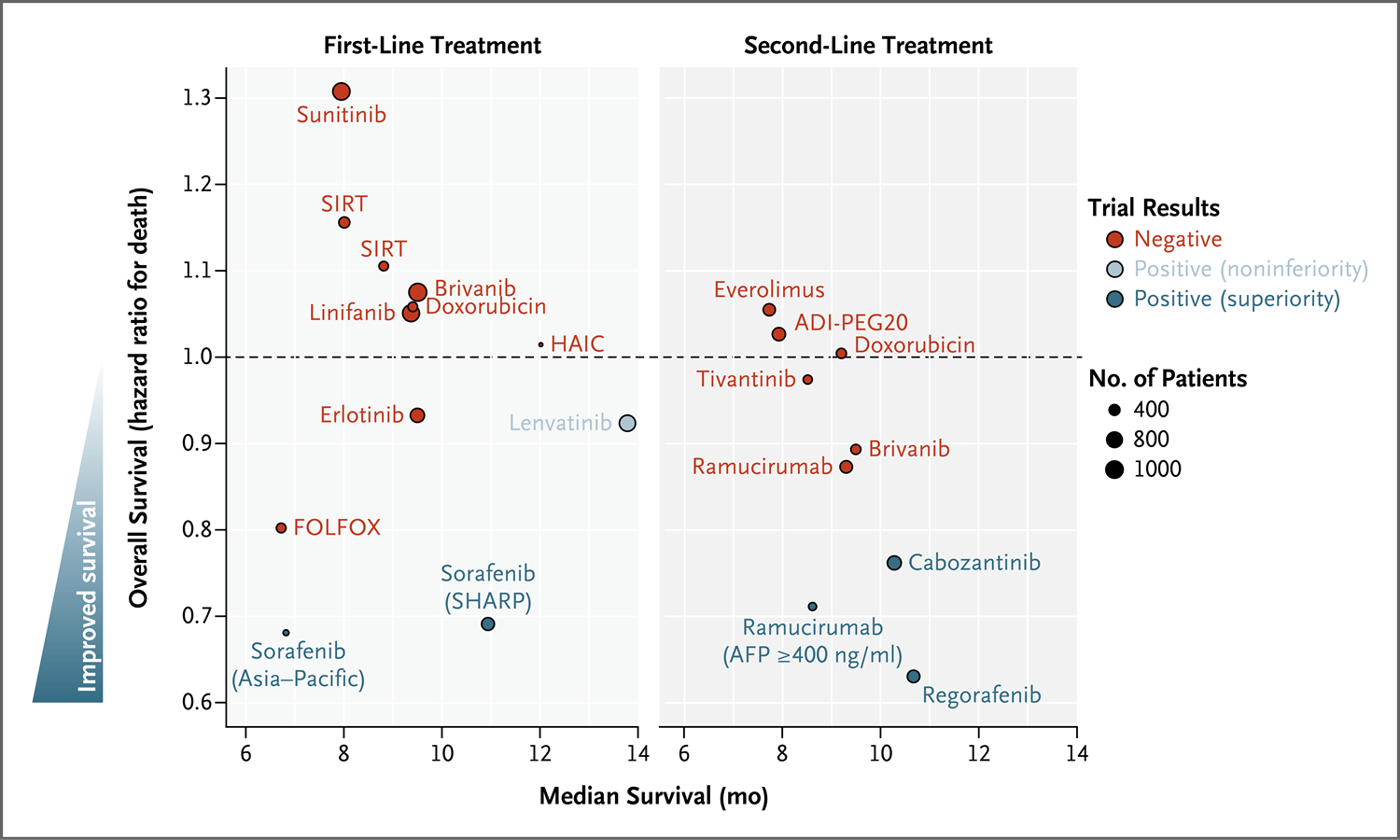
图4.3期试验中评估的晚期肝细胞癌全身性治疗
Figure 4. Systemic Therapies Tested in Phase 3 Trials for the Management of Advanced Hepatocellular Carcinoma.
ADI-PEG20表示聚乙二醇化精氨酸脱亚胺酶-20、HAIC表示肝动脉灌注化疗、SHARP表示评估索拉非尼治疗肝细胞癌的随机方案,SIRT表示选择性体内放疗。
ADI-PEG20 denotes pegylated arginine deiminase 20, HAIC hepatic arterial infusion chemotherapy, SHARP Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol, and SIRT selective internal radiation therapy.
近年来,新的、有效的肝细胞癌全身性治疗试验取得了实质性进展。
In recent years, substantial progress has been made in testing new, efficacious systemic therapies for hepatocellular carcinoma.
瑞戈非尼也是多激酶抑制剂,在索拉非尼治疗期间发生肿瘤进展的患者接受瑞戈非尼治疗后,与安慰剂相比延长了生存期,从7.8个月延长至10.6个月85 。
Regorafenib, also an inhibitor of multiple kinases, increased survival, as compared with placebo, from 7.8 to 10.6 months among patients with tumor progression during treatment with sorafenib.85
瑞戈非尼的安全性与索拉非尼相似,与安慰剂相比,瑞戈非尼使患者死亡风险降低37%,成为美国FDA批准的第一种二线治疗药物。
With a safety profile similar to that of sorafenib, regorafenib decreased the risk of death by 37%, as compared with placebo, and became the first drug approved by the FDA for second-line treatment.
笔记:
- safety profile
在安慰剂对照试验中,作为二线治疗显示出疗效的其他药物包括卡博替尼86 和雷莫芦单抗87 。
Other drugs that have shown efficacy as second-line treatment in placebo-controlled trials are cabozantinib86 and ramucirumab.87
卡博替尼是包括VEGFR、MET和AXL在内的受体酪氨酸激酶抑制剂,与安慰剂相比降低了患者死亡风险(风险比,0.76);卡博替尼和安慰剂组中3或4级不良事件(主要是高血压和掌跖红肿疼痛)的发生率分别为68%和36%。
Cabozantinib, an inhibitor of receptor tyrosine kinases, including VEGFR, MET, and AXL, reduced the risk of death, as compared with placebo (hazard ratio, 0.76); grade 3 or 4 adverse events, mostly hypertension and palmar–plantar erythrodysesthesia, occurred in 68% of patients who received cabozantinib and in 36% of patients who received placebo.
笔记:
- adverse events occurred in结构
雷莫芦单抗试验纳入了基线甲胎蛋白水平≥400 ng/mL的患者。
The ramucirumab trial enrolled patients with baseline alpha-fetoprotein levels of 400 ng per milliliter or greater.
开展本试验的基础是先前一项阴性试验的事后分析结果提示雷莫芦单抗对该亚组有疗效88 。
The trial was based on a post hoc analysis of a previous, negative trial that suggested efficacy in this subgroup.88
笔记:
- 译文的断句值得改善。
VEGF受体-2抗体雷莫芦单抗与安慰剂相比改善了患者生存(死亡的风险比,0.71),且毒性可控。
Ramucirumab, an antibody against VEGF receptor 2, improved survival, as compared with placebo (hazard ratio for death, 0.71), with manageable toxic effects.
笔记:
- manageable toxic effects 毒性可控
肝细胞癌接受免疫治疗的临床获益正在显现。
The clinical benefits of immune-based therapies for hepatocellular carcinoma are emerging.
对细胞毒性T淋巴细胞相关蛋白-4(CTLA-4)抑制剂tremelimumab开展的小规模2期试验表明,患者的部分缓解率为17.6%89 。
A small phase 2 trial with the cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) inhibitor tremelimumab showed a partial response rate of 17.6%.89
在接受索拉非尼治疗后发生疾病进展或不可接受的不良事件的患者中,一项单组2期试验表明,细胞程序性死亡蛋白-1(PD-1)免疫检查点抑制剂纳武利尤单抗使患者达到了15.6个月的中位生存期90,91 。
In patients who had disease progression or unacceptable adverse effects with sorafenib, treatment with the programmed cell death 1 (PD-1) immune checkpoint inhibitor nivolumab achieved a median survival of 15.6 months in a single-group phase 2 trial.90,91
根据《实体肿瘤疗效评价标准》(RECIST),总缓解率为14.3%,此外在有缓解的患者中,55%的缓解持续时间超过12个月92 。
The overall response was 14.3% according to Response Evaluation Criteria in Solid Tumors (RECIST), and in 55% of the patients with a response, the duration of the response was more than 12 months.92
这些缓解数据促使美国FDA依据加速审批项目批准了纳武利尤单抗。
These response data prompted FDA approval under the accelerated program.
另一种PD-1抑制剂帕博利珠单抗的2期试验显示了相似的缓解率(17%),但生存期较短(中位数,12.9个月)93 ;2019年初,帕博利珠单抗二线治疗的相关3期试验并未显示出超过安慰剂的总生存期或无进展生存期(两个主要终点)94 。
A phase 2 trial of pembrolizumab, another PD-1 inhibitor, showed a similar response (17%) but shorter survival (median, 12.9 months)93; in early 2019, the associated phase 3 trial of pembrolizumab for second-line treatment did not show longer overall survival or progression-free survival (the two primary end points) than placebo.94
纳武利尤单抗治疗后的肿瘤缓解与生存期相关91 ,这强调指出需要为疗效选择准确的生物标志物。
The tumor response to nivolumab correlates with survival,91 underscoring the need for accurate biomarkers of the treatment response.
笔记:
- 强调,再次用的是underscore
仔细选择最有可能达到缓解的患者可使其他患者免受不必要的毒性作用。
Careful selection of patients who are most likely to have a response will spare other patients unnecessary toxic effects.
笔记:
- spare
使用PD-1配体-1染色预测纳武利尤单抗治疗后的缓解情况未取得良好效果90 ,肿瘤突变负荷等替代预测因素正在研究中。
The use of PD-1 ligand 1 staining to predict the response to nivolumab has had poor results,90 and alternative predictors, such as tumor mutational burden, are under investigation.
笔记:
- under investigation
靶向治疗联合免疫检查点抑制剂已在1期试验中进行了评估,其中包括仑伐替尼联合帕博利珠单抗95 以及阿特珠单抗联合贝伐珠单抗96 ,缓解率分别为46%和32%。
The combination of targeted therapies with immune checkpoint inhibitors has been tested in phase 1 trials, including lenvatinib plus pembrolizumab95 and atezolizumab plus bevacizumab,96 with response rates of 46% and 32%, respectively.
正在进行中的免疫疗法3期试验将确定它们在肝细胞癌临床治疗中的作用。
Ongoing phase 3 trials testing immune-based therapies will establish their role in the clinical management of hepatocellular carcinoma.
未来展望
Future Perspectives
肝细胞癌导致的死亡人数增多越来越令人担忧3 。
The increase in deaths due to hepatocellular carcinoma is a growing concern.3
人们希望HBV疫苗的普遍接种、HCV感染治愈率的提高和肝癌监测的改进将减轻这一负担。
The hope is that universal HBV vaccination, the increasing cure rates of HCV infection, and improvements in surveillance will reduce this burden.
笔记:
- 这个增译“人们”,有点意思
肝细胞癌是一种复杂的疾病,常与肝硬化相关;需要通过专科门诊的多学科方法来最大限度地影响病程。
Hepatocellular carcinoma is a complex disease, frequently associated with cirrhosis; a multidisciplinary approach in specialized clinics is required to maximally influence the course of the disease.
肝细胞癌的临床治疗在过去10年中有所改进,尤其是晚期患者的治疗。
The clinical management of hepatocellular carcinoma has improved in the past 10 years, particularly for patients at advanced stages.
其他治疗领域仍缺乏有效的干预措施,例如肝硬化患者的化学预防以及切除术或消融术后的辅助治疗。
Other areas of management remain without effective interventions, such as chemoprevention in patients with cirrhosis and adjuvant therapies after surgical resection or ablation.
笔记:
- remain without
3期试验显示疗效的全身性治疗药物不断增多,随之而来的挑战是确定序贯全身性治疗的顺序,以最小的毒性和成本使临床获益最大化。
As the number of systemic agents found to be effective in phase 3 trials continues to grow, the challenge is to determine the order of sequential systemic therapy that maximizes the clinical benefit with minimal toxic effects and cost.
笔记:
- 随之而来,这四个字放的位置让人很舒服
联合治疗及疾病较早期使用全身性治疗药物的前景将构建未来一个时期的肝细胞癌研究计划。
The prospect of combination therapies and the use of systemic drugs at earlier stages will shape research initiatives for hepatocellular carcinoma in the near future.